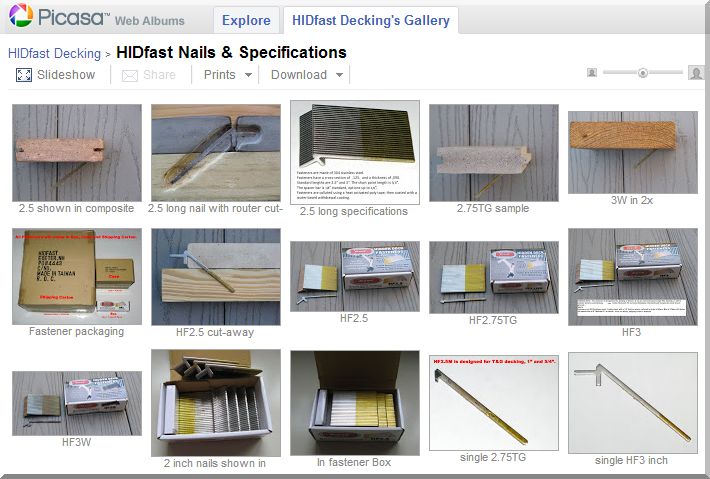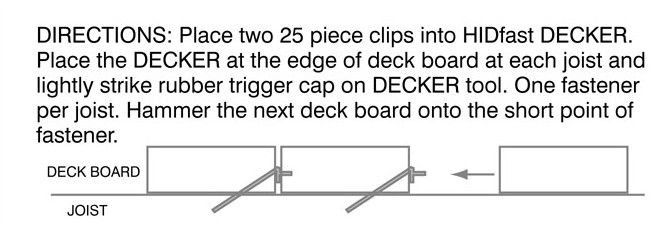
| How stainless steel resists
corrosion
Because stainless steel contains at least 10.5% chromium, the oxidation of the iron is changed to produce a complex oxide that resists further oxidation and forms a passive layer on the surface. This is a very thin layer (microns in thickness) but very tenacious and will reform if it is removed by scratching or machining. The addition of nickel to the structure (8% minimum in 304 and 10% minimum in 316) broadens the range of passivity established by the chromium. The further addition of molybdenum (2% minimum in 316) further expands the passivity range and improves corrosion resistance, notable in acetic, sulfuric, and sulfurous acids and in neutral chloride solutions including sea water. If stainless steel is properly selected and maintained it should not suffer any corrosion. Stainless steel will, however, corrode under certain conditions. It is not the same type of corrosion as experienced by carbon steel. There is no wholesale “rusting” of the surface and subsequent reduction of thickness. If stainless steel corrodes, the most likely form of corrosion is “pitting.” Pitting occurs when the environment overwhelms the stainless steel’s passive film and it cannot heal the interruption. It usually occurs in very tiny dark brown pits on the surface (hence the name pitting), and does not interfere with the mechanical properties of the stainless steel. Stainless steel can also become subject
to crevice corrosion when the deposits or other material (like a washer)
creates a “crevice” on the surface. It is similar to pitting but over a
larger area where again
|
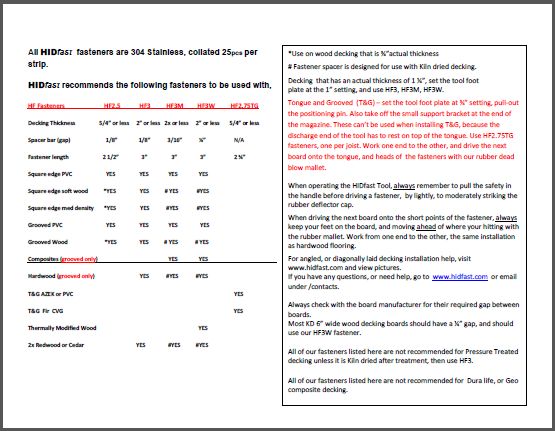 053015
|
|
|